The necessity for fluorescence imaging (FI) to transition from qualitative visual guidance (“the glow”) to Quantitative Fluorescence Imaging (QFI) is paramount for clinical adoption. Metrics like the Tumor-to-Background Ratio (TBR) and Contrast-to-Noise Ratio (CNR) are increasingly vital for diagnostic claims in fields like Fluorescence-Guided Surgery (FGS) especially Molecular Imaging. 1 However, the proliferation of diverse FGS systems and their inherent optical flaws (vignetting, uneven light sources) introduce position-dependent systematic errors, compromising data reproducibility across institutions. 2

The Problem: Non-Uniform Illumination
To ensure consistency, the AAPM Task Group 311 (TG311) published guidelines mandating objective test methods for FGS systems, specifically requiring the characterization of signal detection uniformity and geometric distortion. Without this standardization, the integrity of multi-center clinical trials is jeopardized by unquantified instrumental variance.
Achieving a perfectly homogeneous illumination profile across the Field of View (FOV) is a persistent metrology challenge due to artifacts like Lens Fall-Off (Vignetting), non-uniform LED/Laser Beam Profiles (“spot light effect”), and System Assembly Tolerance (sensor misalignment). These factors mean a true biological signal at the image periphery may be erroneously measured as having lower intensity than an identical signal at the center. This failure compromises TBR integrity and the input validity required for Computer-Aided Detection (CADx) and Artificial Intelligence (AI) applications.
While Flat-Field Correction (FFC) is visually effective, scientific consensus strongly advises caution in quantitative analyses of highly scattering biological tissue. FFC fails because its fixed instrumental map cannot distinguish system non-uniformity from variable signal loss caused by localized tissue properties (scattering and absorption). This often introduces quantitative distortions—such as “overshoot effects”—that negatively impact low-contrast metrics.
The Solution: Constrained Measurement via Uniformity Targets
Given the quantitative unreliability of FFC in heterogeneous media, the established methodology demands a paradigm shift:
- System Mapping: Utilize stable, solid reference phantoms, such as Dot Array Targets, to rigorously characterize the system’s true performance boundaries.
- Constrained Quantification: Quantitative measurements (e.g., TBR) must be restricted and reported only within the demonstrably uniform region of the field of view.
This approach transforms raw, flawed optical data into demonstrably reliable, metrological information by prioritizing robust hardware verification over post-processing, ensuring the integrity necessary for clinical deployment and regulatory submission.

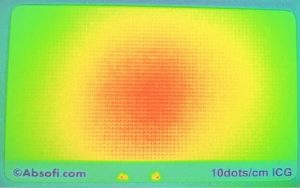
Absofi delivers these dot array targets at an economical price, ensuring that the foundational calibration relies upon a consistently standardized intensity value, thereby guaranteeing the integrity of the long-term spatial calibration matrix. Our fluorescence reference targets provide the ability to quantify performance criteria and align with recently published recommendations by international experts. Read more in the full AAPM TG-311 report 1, or reach out to see how we can help address your needs.. Read more in our blog post on the AAPM TG-311 report, or reach out to see how we can help address your needs.
Disclaimer: This post summarizes the general principles of quantitative medical imaging quality assurance and is not a direct excerpt from the cited article.
- 1: This link refers to the AAPM Task Group Report 311 (TG311): Guidance for performance evaluation of fluorescence-guided surgery systems, which mandates the standardization of FGS system characterization. 2: This link refers to scientific literature discussing the paradox of Flat-Field Correction (FFC), showing that while FFC improves visual quality, it can negatively impact quantitative accuracy in fluorescence imaging.
- 2: This link refers to scientific literature discussing the paradox of Flat-Field Correction (FFC), showing that while FFC improves visual quality, it can negatively impact quantitative accuracy in fluorescence imaging.

The clarity here is outstanding.